k electron affinity|Electron affinity : Pilipinas The electron affinity (Eea) of an atom or molecule is defined as the amount of energy released when an electron attaches to a neutral atom or molecule in the gaseous state to form an anion. X(g) + e → X (g) + energyThis differs by sign from the energy change of electron capture ionization. The electron affinity is positive when energy is released on electron capture. Best 777 Themed Slots. There are so many 777 themed slots available on the market that it might be hard to find the best of the best. That’s why we took the liberty of putting together this list of 3 best 777 slots you can play right here, on SlotsWolf! Mr. Macau by BetSoft. In June 2021, BetSoft came out with this amazing 777 slot. The .
PH0 · electron affinity
PH1 · What is Electron Affinity?
PH2 · What Is Electron Affinity?
PH3 · Lesson Explainer: Electron Affinity
PH4 · Electron affinity: period trend (video)
PH5 · Electron affinity (data page)
PH6 · Electron affinity
PH7 · Electron Affinity Chart (Labeled Periodic table + List)
PH8 · Electron Affinity
PH9 · 7.5: Electron Affinities
A: the "best answer" is completely wrong. ps4 pro is 4k, ps4 slim is not the cheapest, just a newer slimmer- better- model of ps4 and does NOT have the least storage, it can be purchased in 1tb hard drive. Also you need to check the Series number of the specific console if you really want to know how well it will perform because ps4 comes in a .
k electron affinity*******Ago 11, 2023
Electron affinity is defined as the change in energy (in kJ/mole) of a neutral atom (in the gaseous phase) when an electron is added to the atom to .
This page deals with the electron affinity as a property of isolated atoms or molecules (i.e. in the gas phase). Solid state electron affinities are not listed here.k electron affinityThis page deals with the electron affinity as a property of isolated atoms or molecules (i.e. in the gas phase). Solid state electron affinities are not listed here.

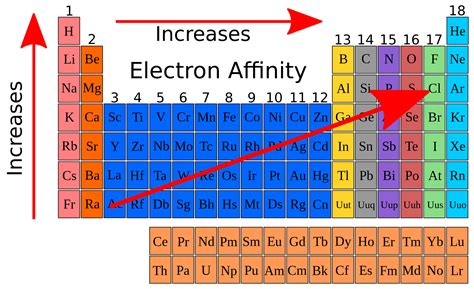
The electron affinity (Eea) of an atom or molecule is defined as the amount of energy released when an electron attaches to a neutral atom or molecule in the gaseous state to form an anion. X(g) + e → X (g) + energyThis differs by sign from the energy change of electron capture ionization. The electron affinity is positive when energy is released on electron capture.The electron affinity (Eea) of an atom or molecule is defined as the amount of energy released when an electron attaches to a neutral atom or molecule in the gaseous state to form an anion. X(g) + e → X (g) + energyThis differs by sign from the energy change of electron capture ionization. The electron affinity is positive when energy is released on electron capture.k electron affinity Electron affinity The chlorine atom has the most negative electron affinity of any element, which means that more energy is released when an electron is added to a gaseous chlorine atom .Chemists define electron affinity as the change in energy, measured in units of kJ/mole, experienced when an electron is added to a gaseous atom. This process creates a negative ion. This process differs from . About. Transcript. Electron affinity is the energy change that results from adding an electron to a gaseous atom. For example, when a fluorine atom in the gaseous state gains an .Electron affinity is defined as. The amount of energy released when an electron is added to a neutral atom to form an anion. The electron affinity is the potential energy change of the atom when an electron is added to .
Vedonlyönti live-vedossa otteluiden aikana. Suorat ja maksuttomat lähetykset VeikkausTV:ssä - pelaa sitä, mitä katsot. Veikkaus. Siirry sisältöön Saavutettavuus. . Uutiset Kuponki Tilastot Voitto-osuudet Live-tilastot. 4.8.2024 Anttoni Huttunen on Ykkösliigan heinäkuun pelaaja.
k electron affinity|Electron affinity